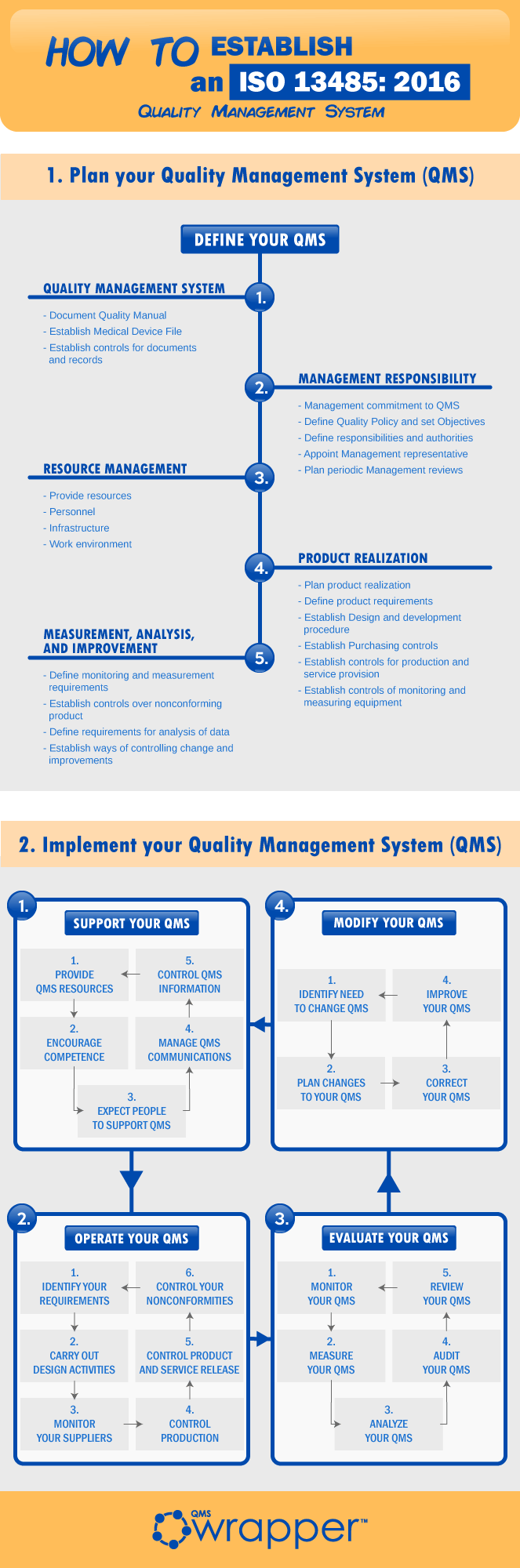
Acquisition of ISO 13485 certification - Total support for medical devices regulatory affairs / SunFlare Japanese
ISO 13485:2016 - Medical devices — Quality management systems — Requirements for regulatory purposes

MD-QMS Measurement, Analysis and Improvement Clause 8 of ISO 13485:2016| Training on ISO 13485:2016| - YouTube

ISO 13485: medical devices - quality management systems - requirements for regulatory purposes | Semantic Scholar

ISO 13485:2016 - Medical devices — Quality management systems — Requirements for regulatory purposes




.gif)