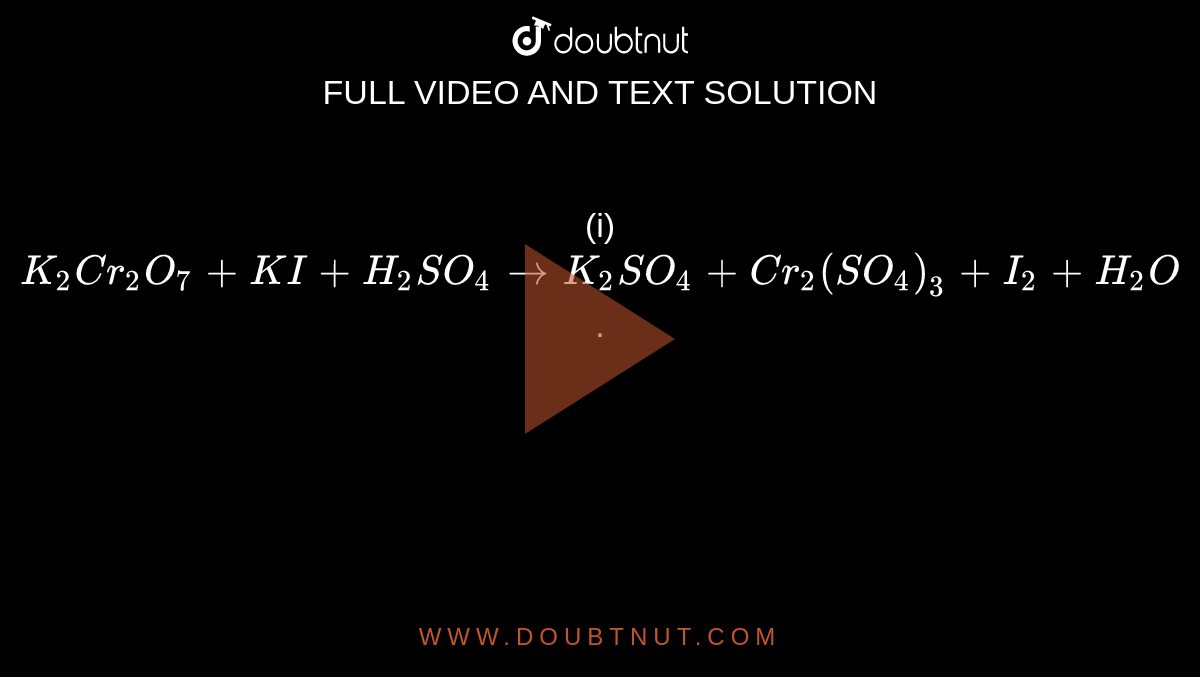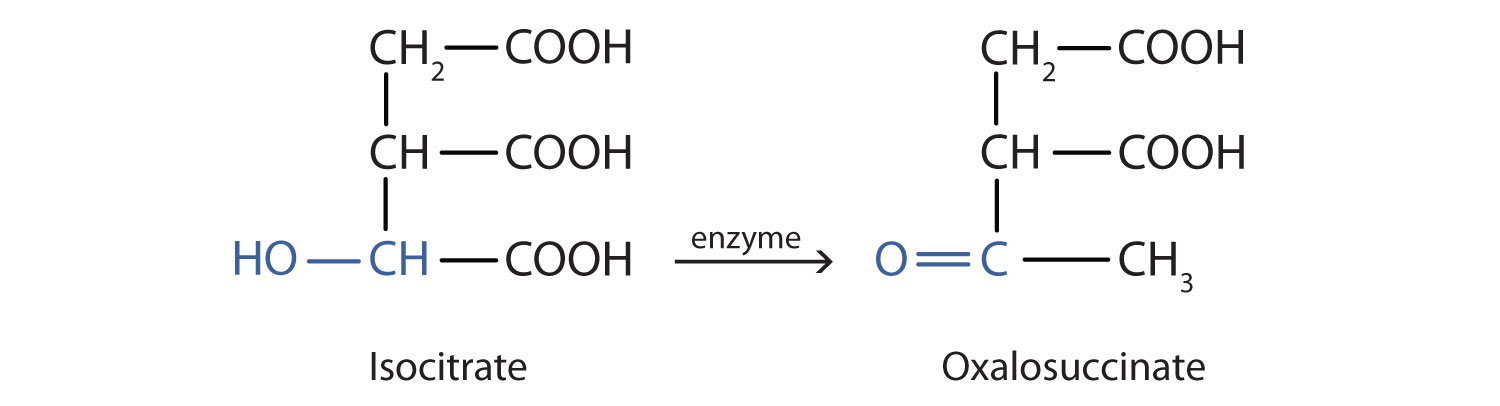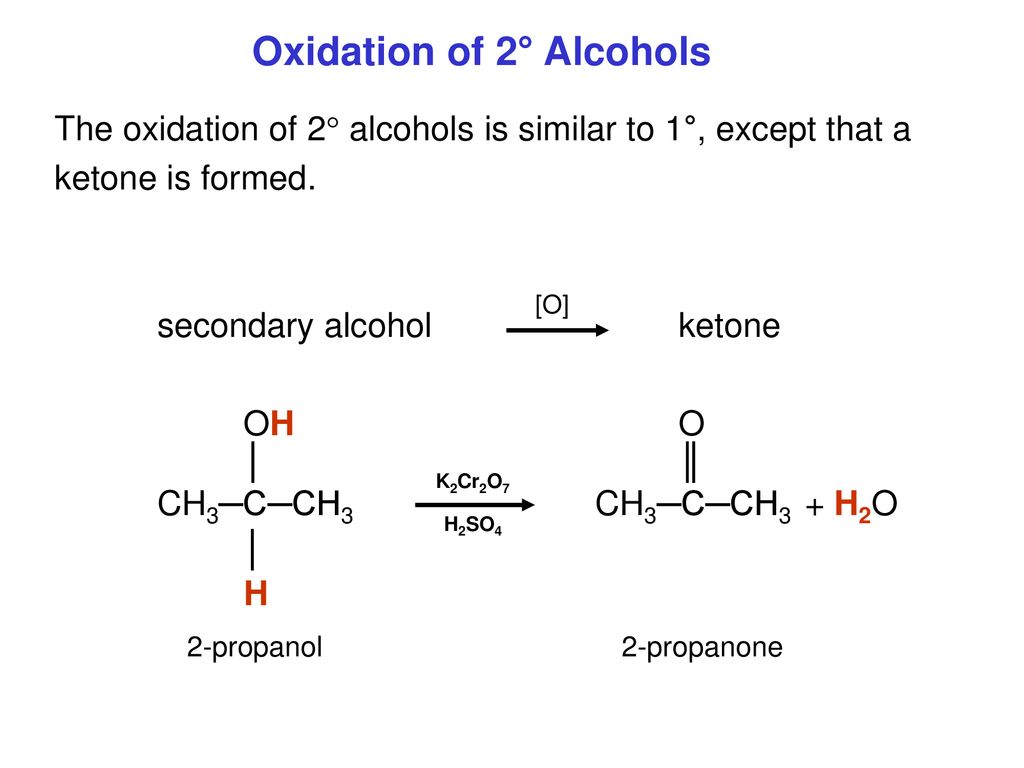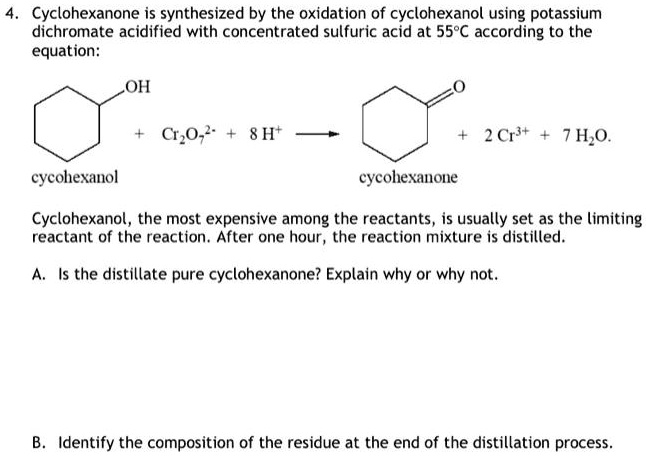
SOLVED: Cyclohexanone is synthesized by the oxidation of cyclohexanol using potassium dichromate acidified with concentrated sulfuric acid at 559C according to the equation: OH C,0z' 8 Ht 2 Crst 7 H,o. cycolexanol
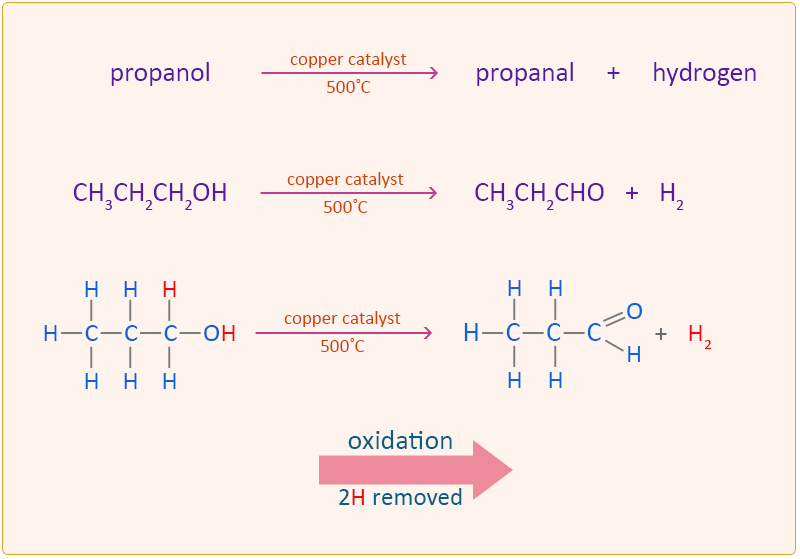
What is the action of acidified K2Cr2O7 on the following : (1) C2H5OH - Sarthaks eConnect | Largest Online Education Community

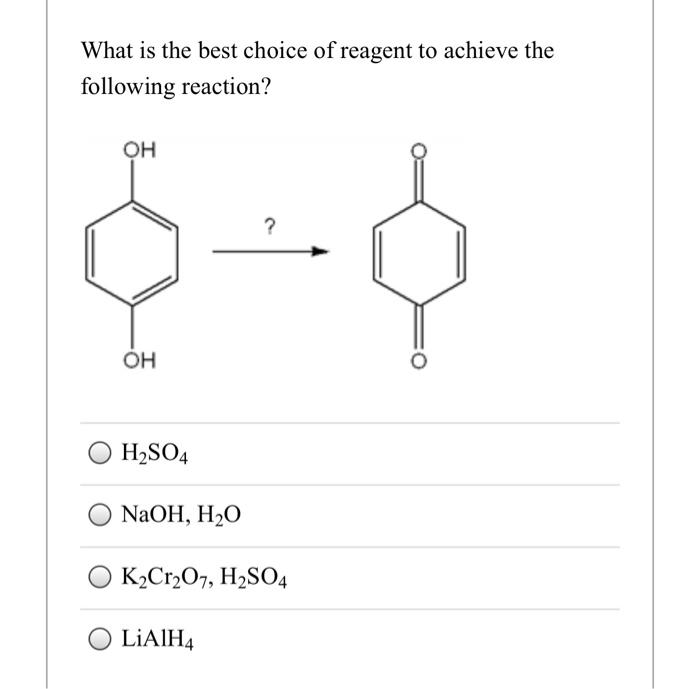
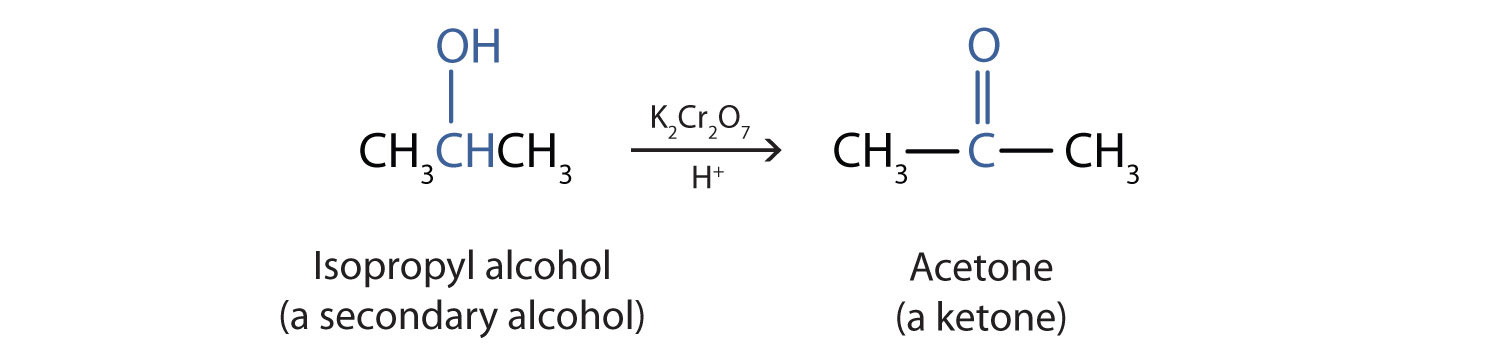
Give the products when is oxidised using following reagents : (i) K(2)Cr(2)O(7) //H(2)SO(4) (ii) CrO(3) // H(2)SO(4) ,acetone (iii) CrO(3)//Pyridine (iv) Pyridinium chlorochromate

Question Video: Determining the Name and Odor of the Product of the Complete Oxidation of Ethanol | Nagwa