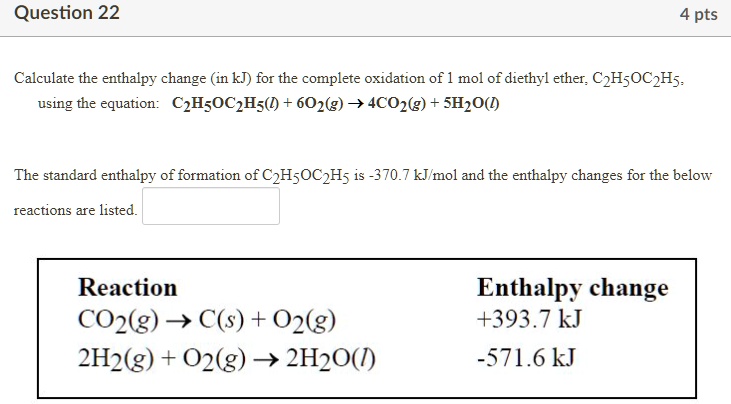
SOLVED: Question 22 pts Calculate the enthalpy change (in kJ) for the complete oxidation of mol of diethyl ether; CzHsOC2Hs: using the equation: C2HsOCHs() + 602kg) + 4CO2(g) + SH2O() The standard

Question Video: Determining a Standard Enthalpy Change Given the Standard Enthlapy of Fusion | Nagwa

Question Video: Determining the Standard Enthalpy of Formation of Ethanol Using Standard Enthalpies of Combustion | Nagwa

thermodynamics - Does the enthalpy of solution formula (LE + Hyd) change depending on the question? - Chemistry Stack Exchange
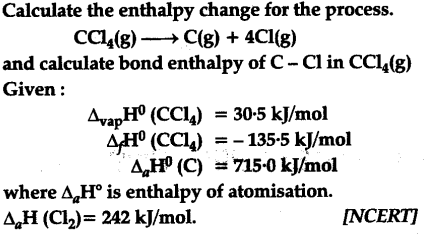
16. Calculate the enthalpy change for the process CCl4(g)————C(g)+4Cl(g) And calculate bond enthalpy of C Cl in CCl4(g). Δ vapH(CCl4)=30.5 kj /mol Δ fH(CCl4)= 135.5 kj/mol Δ aH(C)=715.0 kj/mol Δ aH(Cl2)=242 kj/mol
By the end of today's lesson you should · know what enthalpy is · · · understand the difference between a molar enthalpy v






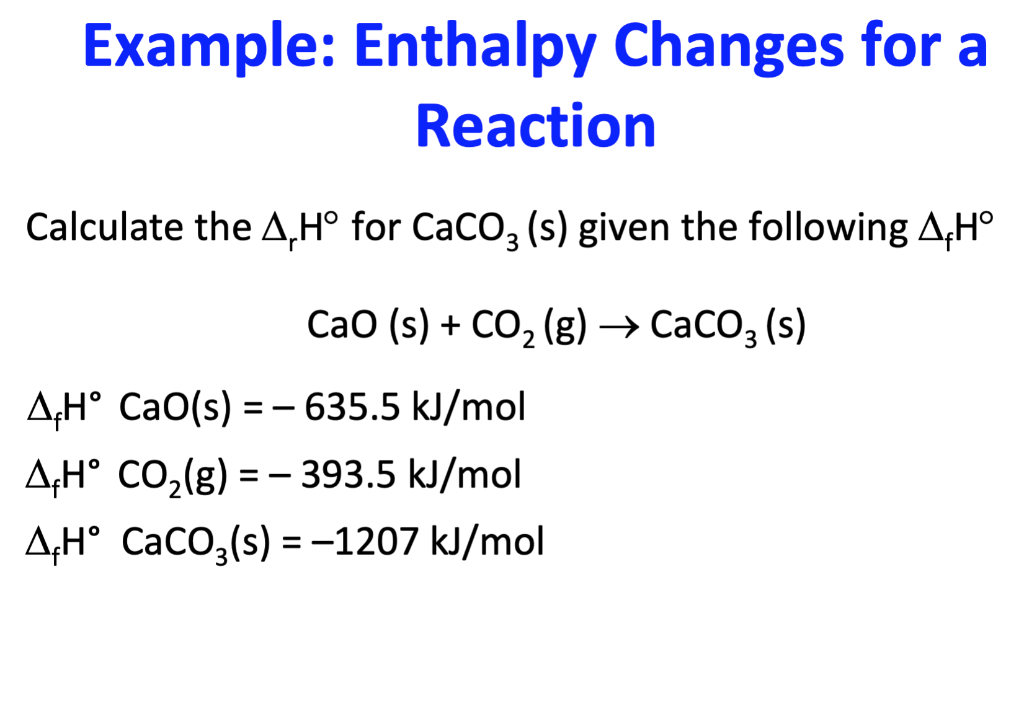



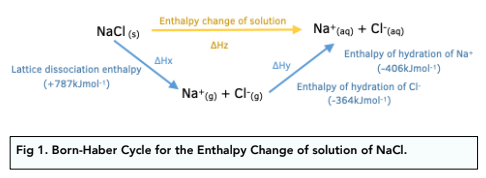
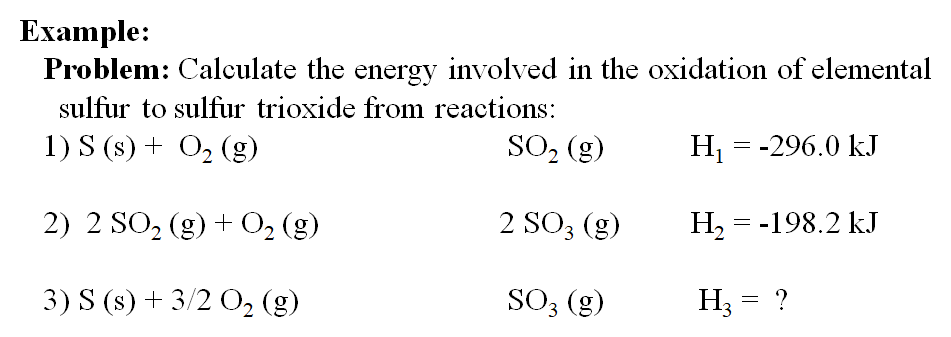
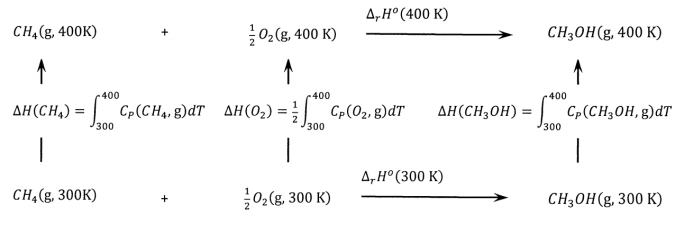
![Example] How to Calculate Enthalpy Change of a Reaction. - YouTube Example] How to Calculate Enthalpy Change of a Reaction. - YouTube](https://i.ytimg.com/vi/nmNQUGt6NiM/maxresdefault.jpg)