Thermal performance evaluation and analysis of helium heat exchanger for cryogenic propellant launch vehicle - ScienceDirect
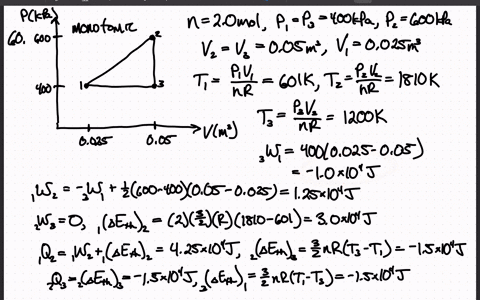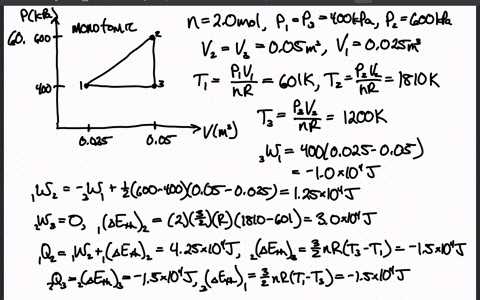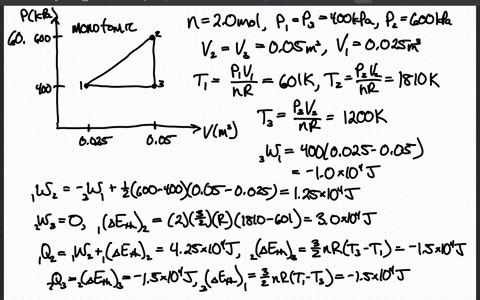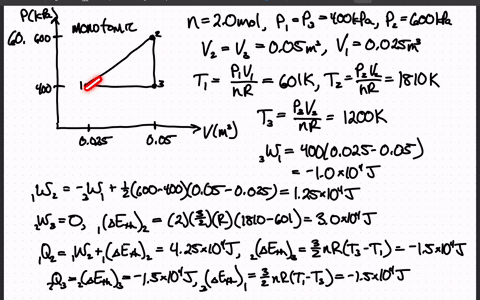
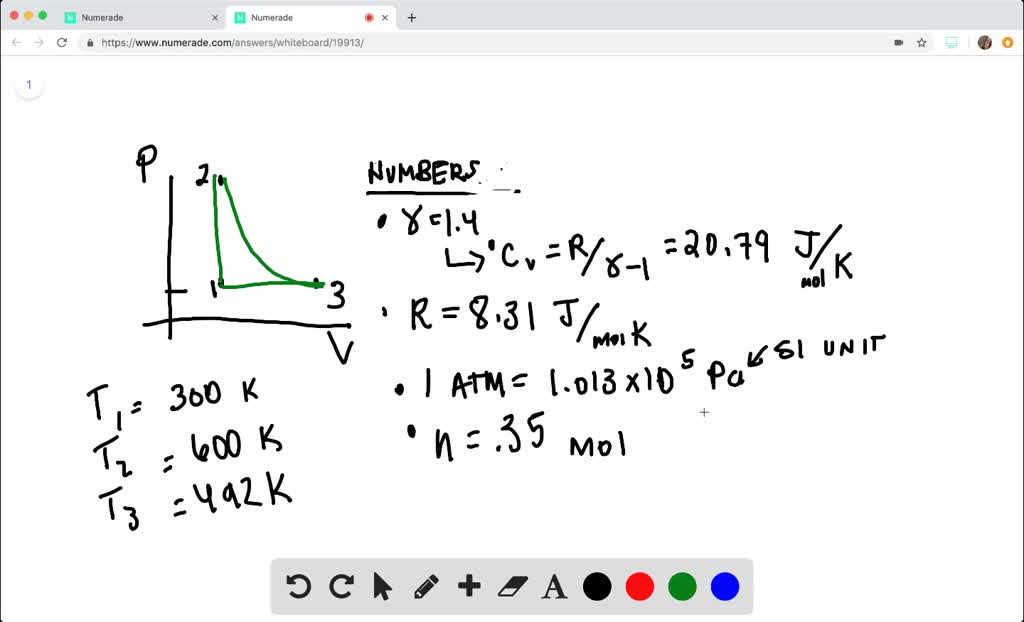
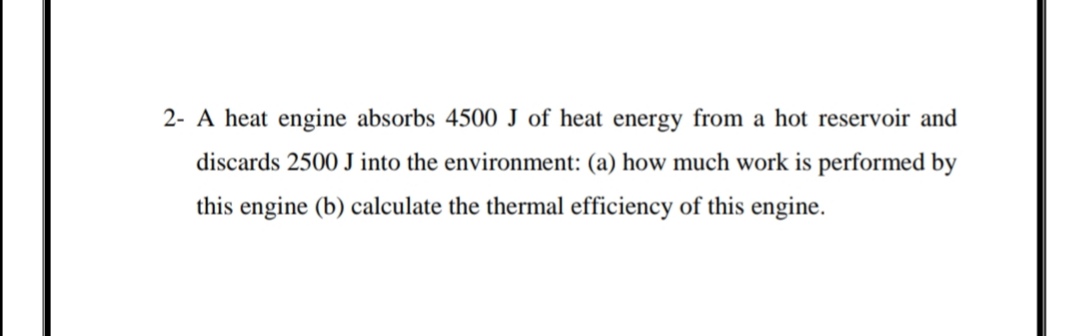
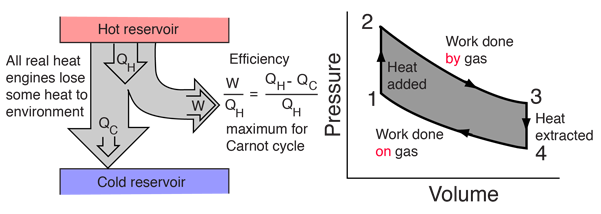
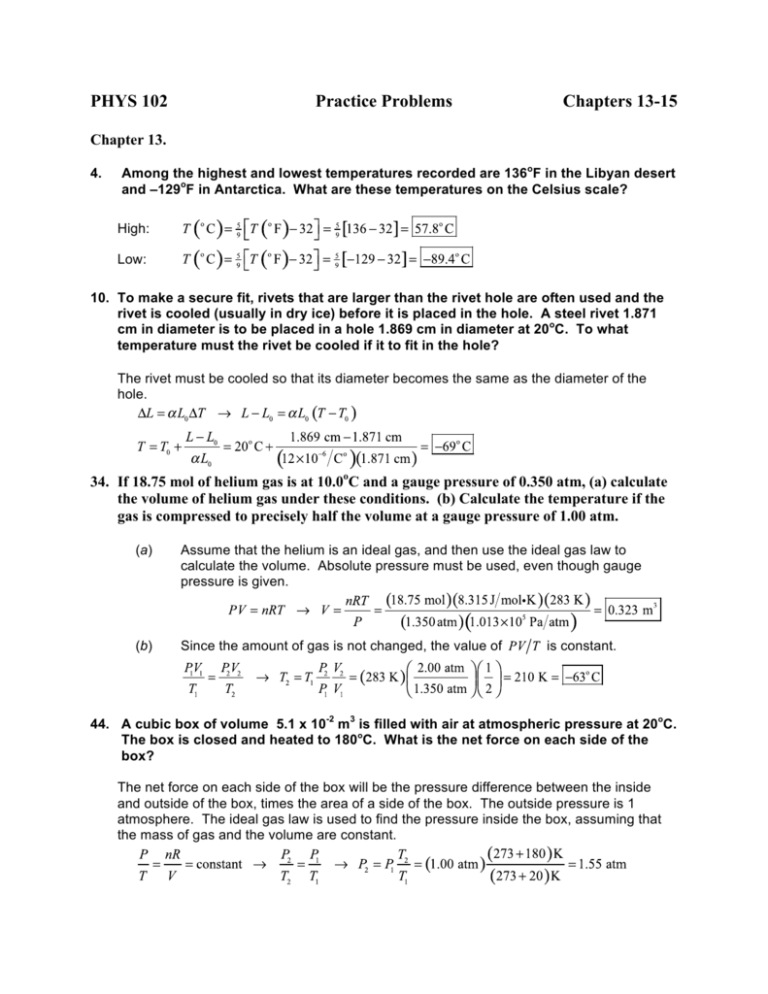
SOLVED:The heat engine shown in HGURE P19.60 uses 2.0 mol of a monatomic gas as the working substance. a. Determine T1, T2, and T3 b. Make a table that shows ΔEth, Ws,
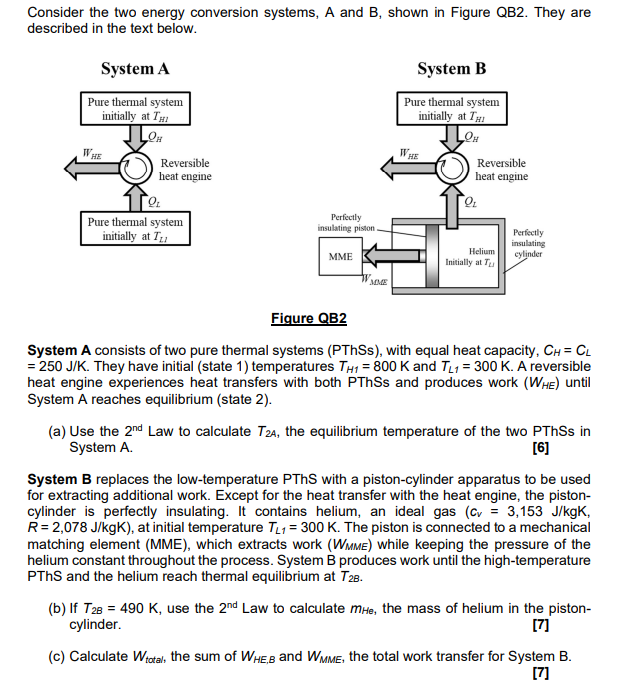
Week 14 Homework Assignment - n moles of helium are initially at a temperature of T0 and occupy a - Studocu

15.14 | Calculate the net work output of a heat engine following path ABCDA in the figure below. - YouTube
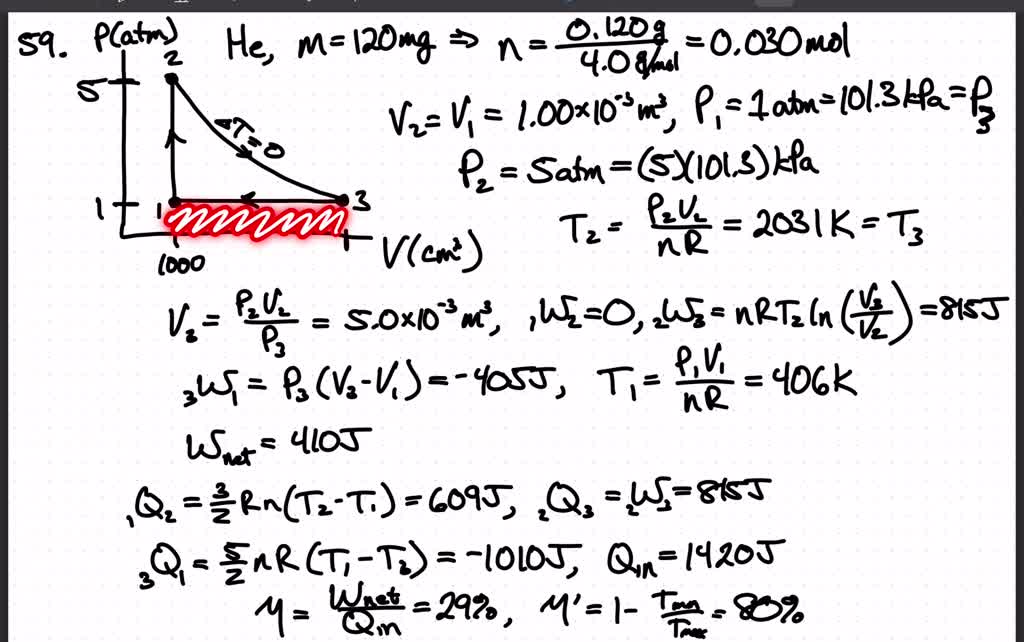
SOLVED:A heat engine using 120 mg of helium as the working substance follows the cycle shown in FIGURE P19.59. a. Determine the pressure, temperature, and volume of the gas at points 1,2,
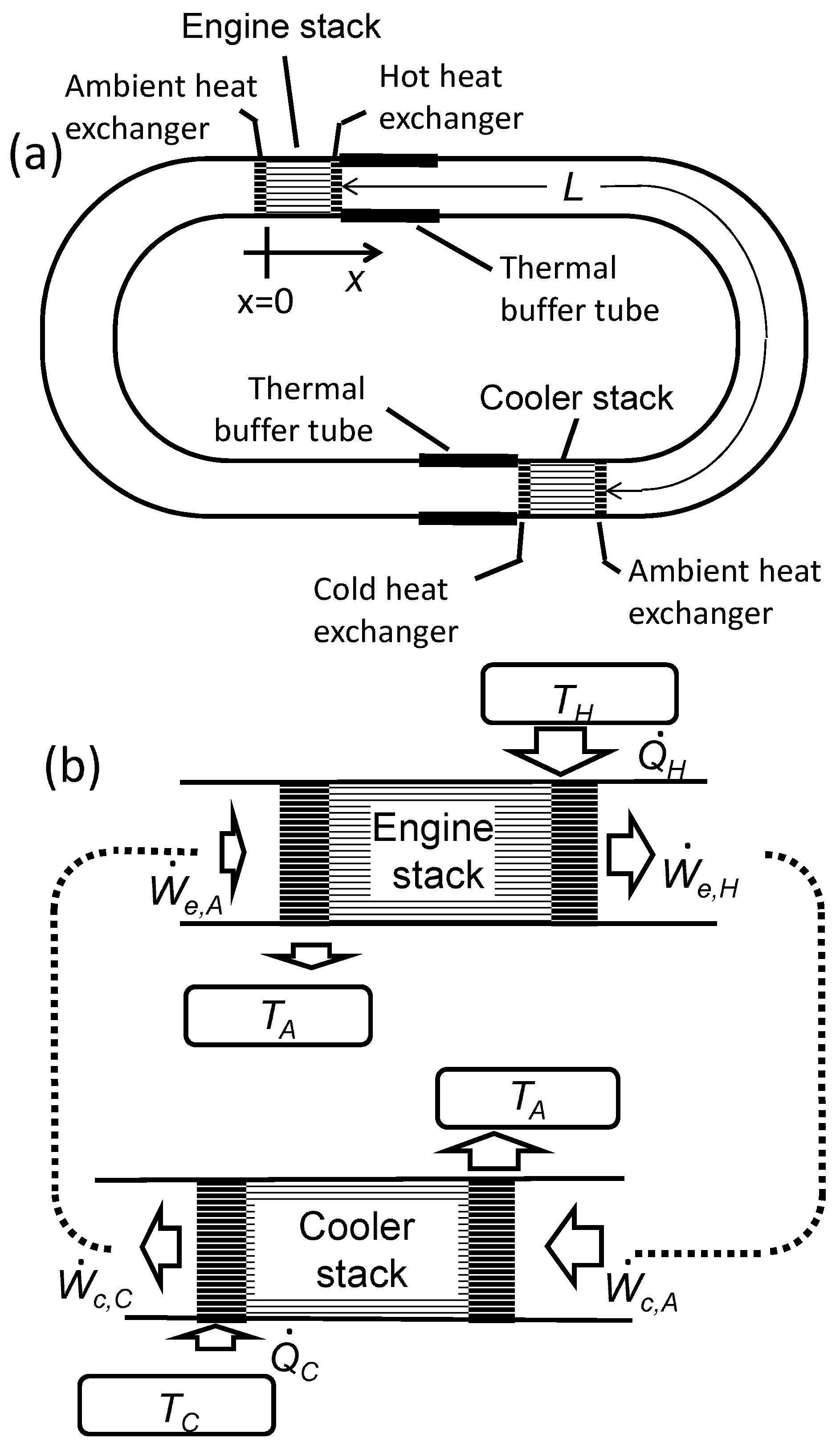
Applied Sciences | Free Full-Text | Numerical Calculation of the Performance of a Thermoacoustic System with Engine and Cooler Stacks in a Looped Tube
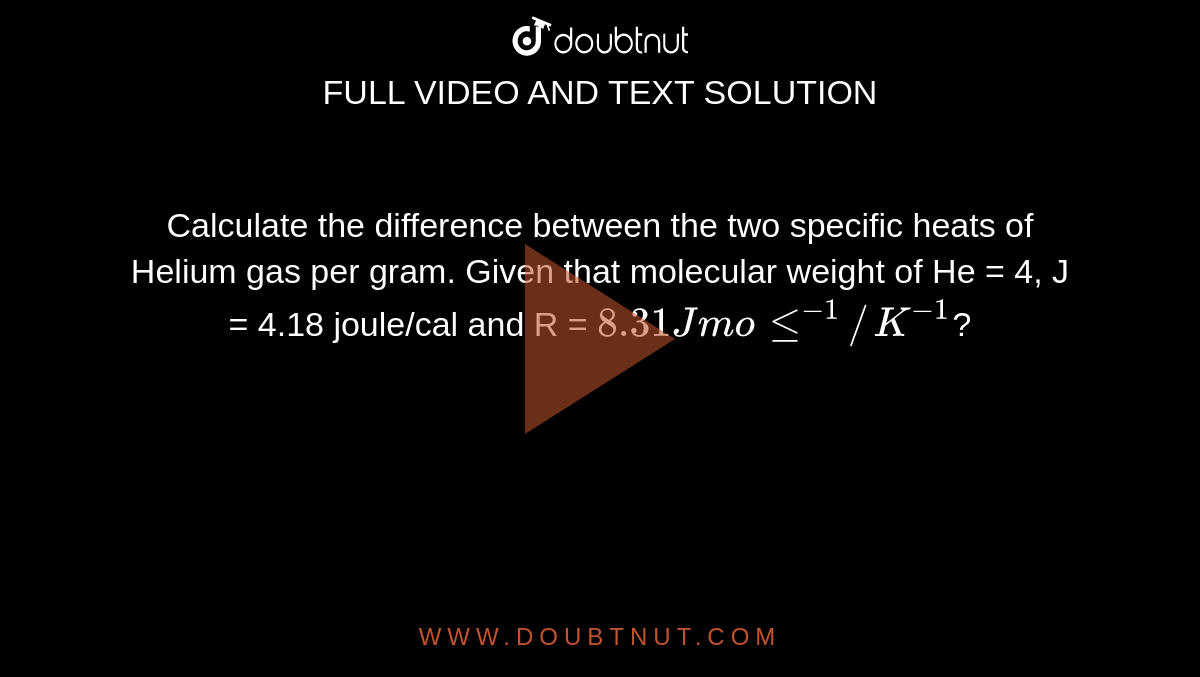
Calculate the difference between the two specific heats of Helium gas per gram. Given that molecular weight of He = 4, J = 4.18 joule/cal and R = 8.31 J mole^-1//K^-1?