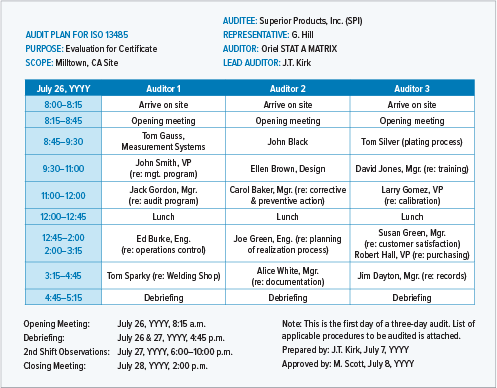
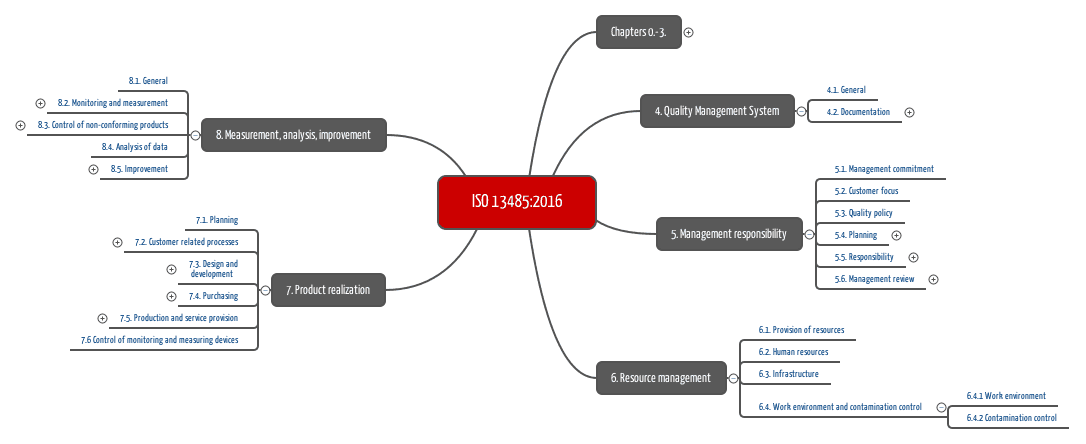
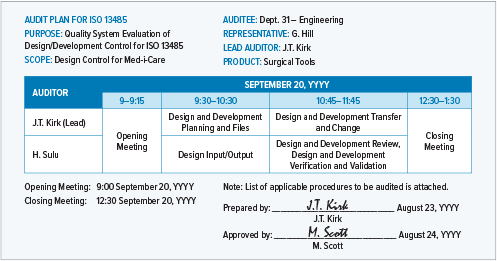
Lead Auditor Training & Certification on MD-QMS Requirements for Regulatory Purposes based on ISO 13485 | TÜV SÜD in India
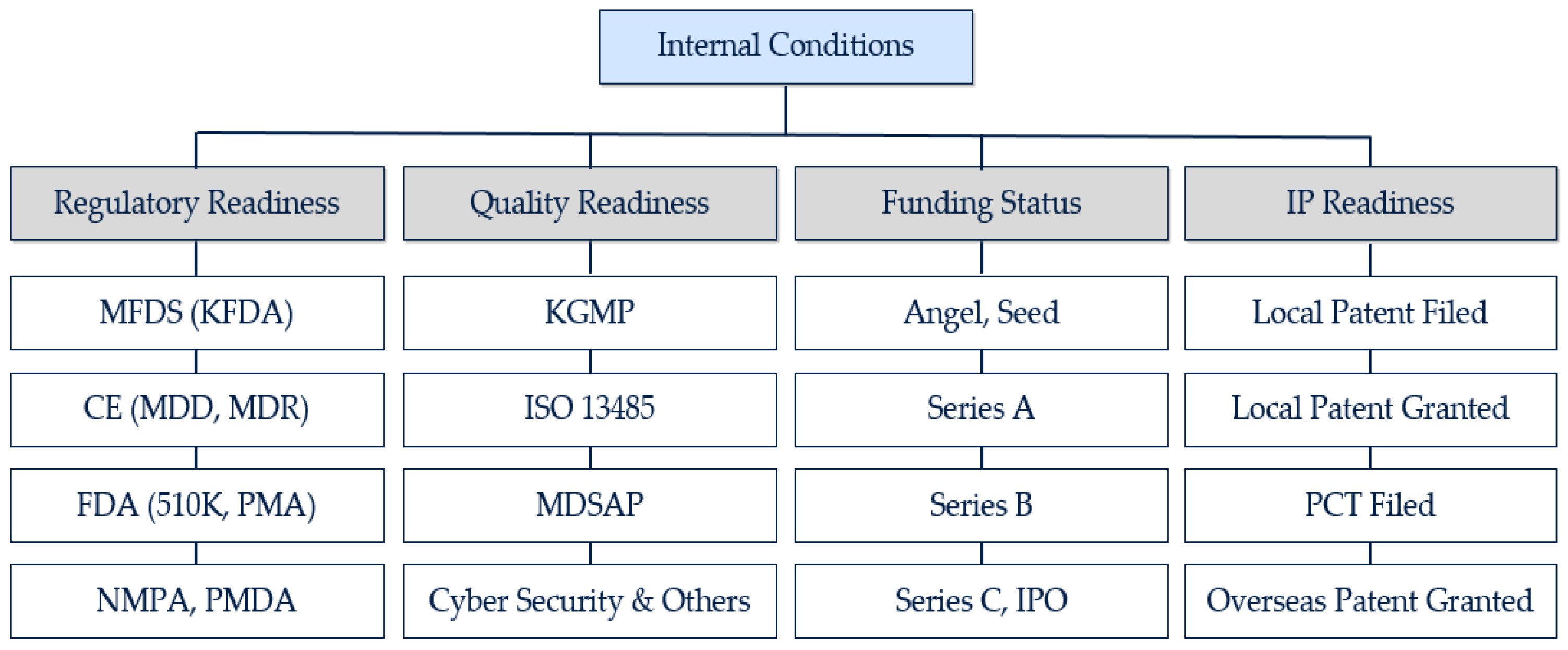
JOItmC | Free Full-Text | What Are the Success Factors for a Partnership with Global Medical Device Companies? Evidence from Korea
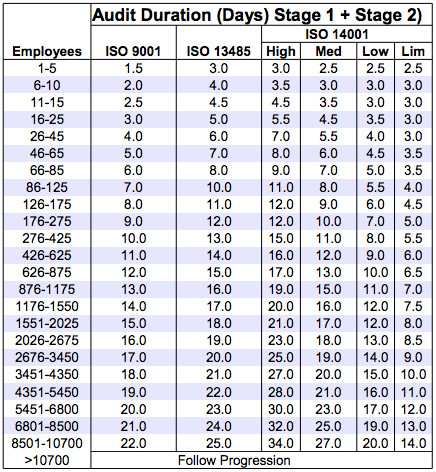
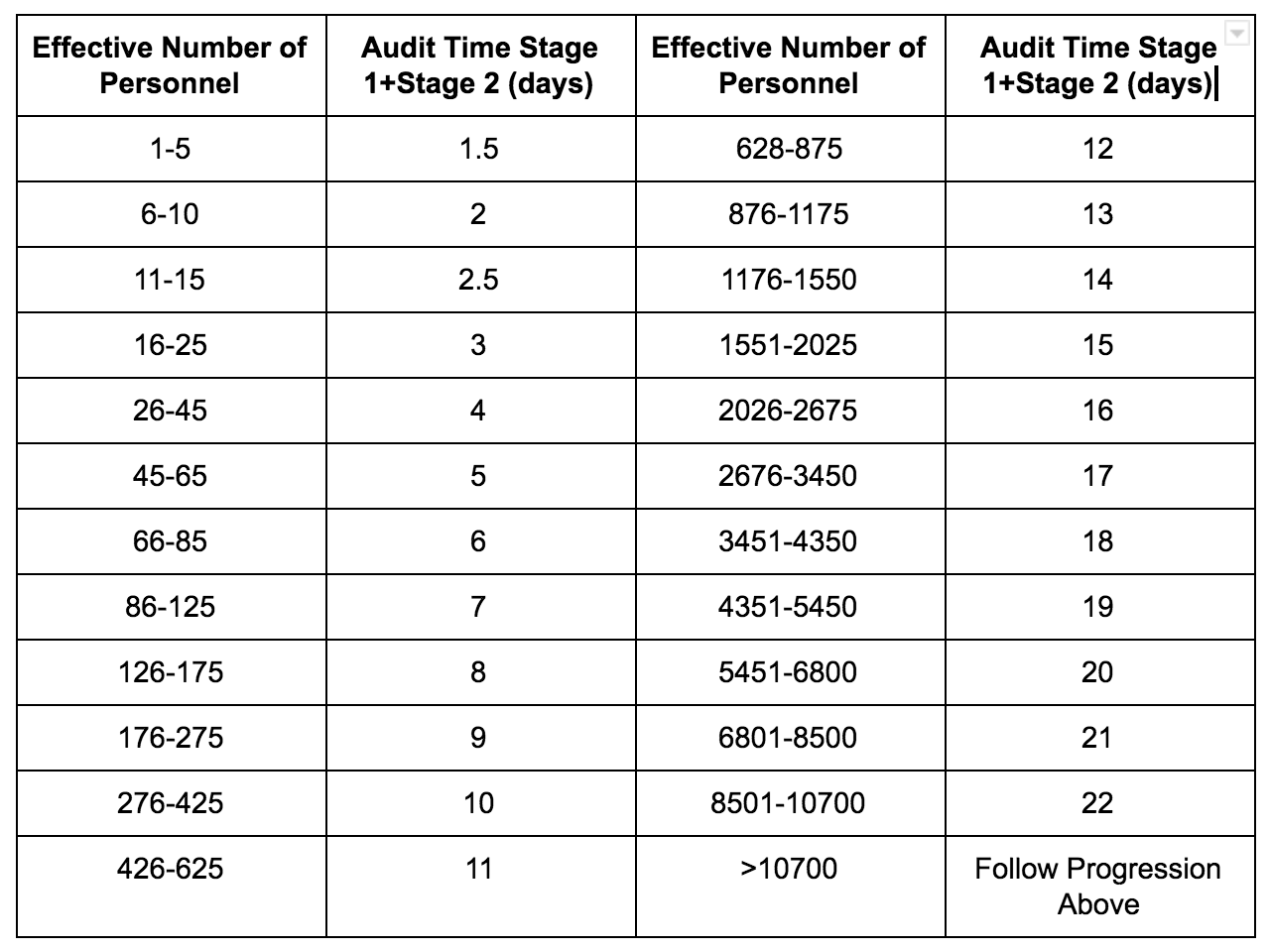
IAF Mandatory Document Determination of Audit Time of Quality and Environmental Management Systems (IAF MD 5: 201X)

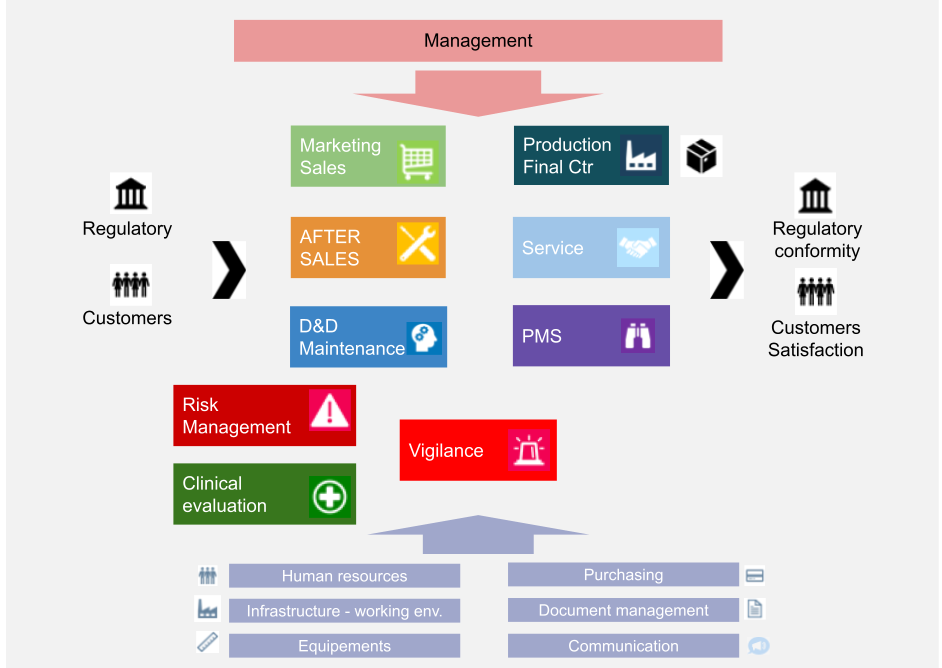
Medical Device Quality Management System Auditor (ISO 13485) - Certus Professional Certification Inc.